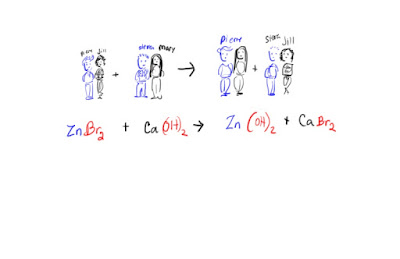TEACHING NOTES:
First lets review the main types of reactions:
Chemical reactions may be categorized as follows :
1. Synthesis
2. Decomposition
3. Single replacement
4. Double replacement
5. Combustion
Cellular Respiration
6. Oxidation of metals
A Synthesis reaction:
element + element --> compound
Decomposition reaction:
Compound --> element + element
Or
Compound --> smaller compound + element
IN ADDITION TO THE FOUR TYPES ABOVE, THERE ARE ALSO THREE MORE KINDS of reaction TO KNOW:
Combustion: combustion may happen to any *organic compound* meaning any compound made of carbon, hydrogen and/or oxygen...
Carbon based compound+ oxygen --> carbon dioxide + water
+ heat and light energy
Cell respiration looks like combustion,
BUT IT IS NOT. YOUR CELLS ARE NOT COMBUSTING. The carbon based compound in this case is glucose, a simple sugar.
Glucose + oxygen --> carbon dioxide + water + energy for cell
Oxidation reaction
Metal + oxygen --> metal oxide
ACID BASE REACTIONS ARE ALL NEUTRALIZATION REACTIONS
Metal + oxygen --> metal oxide
ACID BASE REACTIONS ARE ALL NEUTRALIZATION REACTIONS
In summary....