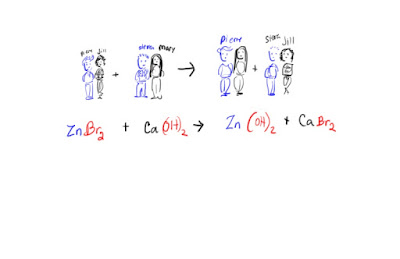Recall that worksheet Chemistry practice sheet and the Chemistry practice sheet key. This was given for homework and you will be tested on this soon.
if you are done that, then there is another worksheet on types of reactions:
worksheet. Here is the answer key for types of reactions. Please write out these answers in your notebook and show me when done for work period marks. There is a typo on this one:
Section 2 number 3 has a typo on answer key. The co-efficients should be...4, 5, 4,6 and not what they have on the key
Number 3 section 3 is 1,2,2
Monday 16 October 2017
Thursday 12 October 2017
Types of Chemical Reactions
Chemical
reactions may be categorized as follows
1.
Synthesis
2.
Decomposition
3.
Single replacement
4.
Double replacement
5. Combustion
6. Oxidation of metals
A Synthesis reaction:
element +
element --> compound
Decomposition reaction:
Compound --> element +
element
Or
Compound --> smaller compound + element
Combustion
Carbon based
fuel + oxygen --> carbon dioxide + water
Cell respiration looks like combustion,
BUT IT IS
NOT. YOUR CELLS ARE NOT COMBUSTING
IN THE MITOCHONDRIA
Glucose + oxygen --> carbon dioxide + water + energy for cell
balancing lego equations, practice sheets and types of reactions
We practiced balancing equations using lego. make sure you finish this and hand it in to msngscience@gmail.com
IF you are finished, practice your chemistry:
Recall that worksheet Chemistry practice sheet and the Chemistry practice sheet key. This was given for homework and you will be tested on this soon.
if you are done that, then there is another worksheet on types of reactions:
worksheet. Here is the answer key for types of reactions
IF you are finished, practice your chemistry:
Recall that worksheet Chemistry practice sheet and the Chemistry practice sheet key. This was given for homework and you will be tested on this soon.
if you are done that, then there is another worksheet on types of reactions:
worksheet. Here is the answer key for types of reactions
Tuesday 10 October 2017
Calcium and Water lab write up
We did notes on the Law of Conservation of Mass and we did a lab. This is the criteria for the lab write up
Your lab must include the following
1. First and Last names of all investigators
2. Purpose of lab: write about how this lab illustrates the law of conservation of mass. Explain how the lab relates to the law
3. Materials: write a complete list of materials that we used
4. Procedure: write a step by step procedure (including illustrations if it makes it clearer) that is so well written that another person could read it and replicate your actions exactly
5. What is the question we are trying to answer? Write the two chemistry equations which were our possible outcomes of this reaction? Oxygen gas or Hydrogen gas?
6. Results: Describe in detail what happened after you did the procedure. Include any photos you took. How did you know you made the gas, which gas? And was it Calcium hydroxide that was made? How do you know?
7. Write your conclusion in paragraph form.
EVALUATION IS OUT OF 25 IN TOTAL
5
presentation is out of 5. for 5/5 it is typed with illustrations or photos and emailed to msngscience@gmail.com. for 4/5 it is pretty good but there are a few spelling mistakes. for 0-3 out of 5 it is in pencil or messy
10
Clear writing is out of 10. for 9/10 or 10/10, it is written with no English mistakes and it clearly communicates each part of the lab report. 7-8/10 it is very well written for 5-6/10 some parts are not so clear and the reader must guess what you are trying to say. 0-4/10, it is minimally communicating what happened
10
Paragraphs are insightful and your observations are well done. this is out of 10.
9-10/10, your observations and conclusions are well written and logical. It is brilliant
7-8/10 your observations and conclusions are very well done
5-6 your conclusions don't really logically follow from your results
0-4 your conclusions are not relating to your results at all
ACKNOWLEDGEMENTS
write all names and who contributed what including the % of their contribution. so two people who contributed equally would be50%
Your lab must include the following
1. First and Last names of all investigators
2. Purpose of lab: write about how this lab illustrates the law of conservation of mass. Explain how the lab relates to the law
3. Materials: write a complete list of materials that we used
4. Procedure: write a step by step procedure (including illustrations if it makes it clearer) that is so well written that another person could read it and replicate your actions exactly
5. What is the question we are trying to answer? Write the two chemistry equations which were our possible outcomes of this reaction? Oxygen gas or Hydrogen gas?
6. Results: Describe in detail what happened after you did the procedure. Include any photos you took. How did you know you made the gas, which gas? And was it Calcium hydroxide that was made? How do you know?
7. Write your conclusion in paragraph form.
EVALUATION IS OUT OF 25 IN TOTAL
5
presentation is out of 5. for 5/5 it is typed with illustrations or photos and emailed to msngscience@gmail.com. for 4/5 it is pretty good but there are a few spelling mistakes. for 0-3 out of 5 it is in pencil or messy
10
Clear writing is out of 10. for 9/10 or 10/10, it is written with no English mistakes and it clearly communicates each part of the lab report. 7-8/10 it is very well written for 5-6/10 some parts are not so clear and the reader must guess what you are trying to say. 0-4/10, it is minimally communicating what happened
10
Paragraphs are insightful and your observations are well done. this is out of 10.
9-10/10, your observations and conclusions are well written and logical. It is brilliant
7-8/10 your observations and conclusions are very well done
5-6 your conclusions don't really logically follow from your results
0-4 your conclusions are not relating to your results at all
ACKNOWLEDGEMENTS
write all names and who contributed what including the % of their contribution. so two people who contributed equally would be50%
Tuesday 3 October 2017
Chemistry Practice for Chem Charate!!
A really useful Chemistry practice sheet Here is the Chemistry practice sheet key. Note there is another quiz next period
Subscribe to:
Posts (Atom)