Friday, 20 November 2015
Tuesday, 13 October 2015
Wednesday, 30 September 2015
Thursday, 17 September 2015
Welcome to science 9
In today's class you will create safety rules by reading and summarizing the safety procedures in your text. Then you will take the Finn Laboratory Saftey Test
The rules can be under the following categories:
HIGHEST PRIORITIES:
COMMUNICATE AND REACT TO AN EMERGENCY
Talk to the instructor Emergency procedures for fire, toxins, spills, evacuation.
KEEP ORGANIZED AND SAFE
Keep the classroom safe
Keeping your work area safe
Keep safe with your colleagues
Keep yourself safe
KNOW THE SYMBOLS
Know the correct symbols, WHIMIS symbols
The rules can be under the following categories:
HIGHEST PRIORITIES:
COMMUNICATE AND REACT TO AN EMERGENCY
Talk to the instructor Emergency procedures for fire, toxins, spills, evacuation.
KEEP ORGANIZED AND SAFE
Keep the classroom safe
Keeping your work area safe
Keep safe with your colleagues
Keep yourself safe
KNOW THE SYMBOLS
Know the correct symbols, WHIMIS symbols
Monday, 8 June 2015
Remaining Term Assignments
1. Make Two Column notes directly related to doing Ministry Exam corrections. (25 MARKS) that is:
a. Do the four practice exams on the ministry website. Select "Sample Exams"
b. call up the answer keys to these exams and DO THEM.
c. If there is a key word you don't understand in a question you get wrong, include this in a set of Two column notes. For example if you get a question wrong due to the fact that you didn't know what "mutualism" is, your notes should say
mutualism - a form of symbiosis where both organisms benefit
2. Make summary notes (your own) for BIOLOGY, CHEMISTRY, RADIATION, PHYSICS, GEOLOGY (25 MARKS)
3. REDO these exams as an open book exercise (due Tuesday) for full marks for this term: The links will be live tonight.
Biology chapter 1 and 2
Biology chapter 3
Chemistry exam
4. (optional) Camosun bog assignment (40 marks)
a. Do the four practice exams on the ministry website. Select "Sample Exams"
b. call up the answer keys to these exams and DO THEM.
c. If there is a key word you don't understand in a question you get wrong, include this in a set of Two column notes. For example if you get a question wrong due to the fact that you didn't know what "mutualism" is, your notes should say
mutualism - a form of symbiosis where both organisms benefit
2. Make summary notes (your own) for BIOLOGY, CHEMISTRY, RADIATION, PHYSICS, GEOLOGY (25 MARKS)
3. REDO these exams as an open book exercise (due Tuesday) for full marks for this term: The links will be live tonight.
Biology chapter 1 and 2
Biology chapter 3
Chemistry exam
4. (optional) Camosun bog assignment (40 marks)
Monday, 25 May 2015
Do your corrections for the quizzes below
So you are ready for the retest. I will be available lunch hours this week
Physics quiz
and
Radioactivity quiz
Your corrections must be done to do the retest of the same concepts
Physics quiz
and
Radioactivity quiz
Your corrections must be done to do the retest of the same concepts
Thursday, 14 May 2015
Starting our Geology Unit
The following resources would be useful to you: Your homework is to start working on this
Your notes for the unit are here: We will go over these in class.
Your notes for the unit are here: We will go over these in class.
-and older exams which include many of the topics from our unit.
the provincial exam study package, do chapter 12 section only. chapter 10 and 11 are no longer on the provincial. answer key at the end of the package.
Tuesday, 12 May 2015
full science notes, including radioactive decay
your notes may be downloaded here
Please get ready for a test next period on Radioactivity. Your worksheet from the last post ought to be complete and your answers checked. Practice the provincial exam questions on radioactivity.
Please get ready for a test next period on Radioactivity. Your worksheet from the last post ought to be complete and your answers checked. Practice the provincial exam questions on radioactivity.
Monday, 4 May 2015
Radiation Unit
Today we will have a lesson on Alpha Beta and Gamma decay and do some practice questions.
Notes can be downloaded here and answer key to the questions can be accessed here.
Keep in mind that your radiation test will occur next thursday.
Notes can be downloaded here and answer key to the questions can be accessed here.
Keep in mind that your radiation test will occur next thursday.
Friday, 17 April 2015
Tuesday, 14 April 2015
Physics Unit: Motion, Velocity
Please ensure you have the following items:
1. diagrams for our physics unit
2. graph your data of our skateboard/number line demo (April 8)
3. Practice questions from provincial exam study guide for motion. (April 6)
4. Workbook study questions from April 10: You may check your answers here
And a situation where one's life depended on the physics of motion : Apollo 13
1. diagrams for our physics unit
2. graph your data of our skateboard/number line demo (April 8)
3. Practice questions from provincial exam study guide for motion. (April 6)
4. Workbook study questions from April 10: You may check your answers here
And a situation where one's life depended on the physics of motion : Apollo 13
Monday, 9 February 2015
Practice and more practice
Do the some useful practice worksheets copyright Ms. Meer: and the answer key for molecular compounds. The safety test will be postponed to thursday.
Wednesday, 4 February 2015
science conference opportunity
Dr. David Ng is organizing a science extravaganza filled with lecture, demos and hands on interaction with science stuff:
MOST EXCEPTIONAL ESCAPADES IN SCIENCE CONFERENCE
I would like to register students from my four classes on a first come first serve basis. We may need to collect a fee for a sub (about $15) but I'll attempt to get that waved.
Let me know if you are interested!
MOST EXCEPTIONAL ESCAPADES IN SCIENCE CONFERENCE
I would like to register students from my four classes on a first come first serve basis. We may need to collect a fee for a sub (about $15) but I'll attempt to get that waved.
Let me know if you are interested!
Monday, 2 February 2015
Safety Test
We will be having a safety test in preparation for doing chemistry labs. Please read through the Laboratory Safety Test from Finn Scientific . Read through the safety instructions in your science text. and Create a Safety Poster : Your poster ought to cover major themes including but not limited to the following topics:
- instructions
- personal safety
- personal classroom space, shared classroom space
- group safety
- first aid
- fire
- MSDS labels
Your safety poster ought to have fewer than 7 main rules which cover all the topics. (14 marks) It ought to be creative (3 marks) and eye catching and colourful (3 marks) Colour need not be from a colour printer. I have lots of lovely colouring felts in class for you to use!! . It is worth 20 marks
DUE DATE is FEB 10. Bonus if you make a safety vid.
- instructions
- personal safety
- personal classroom space, shared classroom space
- group safety
- first aid
- fire
- MSDS labels
Your safety poster ought to have fewer than 7 main rules which cover all the topics. (14 marks) It ought to be creative (3 marks) and eye catching and colourful (3 marks) Colour need not be from a colour printer. I have lots of lovely colouring felts in class for you to use!! . It is worth 20 marks
DUE DATE is FEB 10. Bonus if you make a safety vid.
Practicing Lewis Diagrams and a Review of Chemical Nomenclature
 |
| image from http://www.chino.k12.ca.us/cms/lib8/CA01902308/Centricity/Domain/1366/dotstructureandtheperiodictable.pdf |
Topic 1. Write the Bohr diagram, the Lewis diagram and tell me the number of Protons, Electrons and Neutrons of any atom. We used this worksheet to practice (copyright T. Trimpe, 2002).
Here is a nice summary (copyright Chino Valley Unified School District) of what we learned so far.
Topic 2. We also learned how to write any ionic compound and also any covalent compound. Below you will find a useful video summary of how to do ionic and covalent compounds.
Topic 3; We did some practicing of grade nine nomenclature stuff. Here are some useful practice worksheets copyright Ms. Meer: and the answer key
Wednesday, 21 January 2015
Why atoms combine and the love song of Sodium Chloride
We had a series of lessons explaining why atoms combine. (bohr diagram worksheets, make sure you get them from me!)
We learned the skill of how to write Bohr diagrams for atoms and for ions. And we did section 4.1 of the study guide for homework. Note the answer key at the end of the guide.
Try this practice quiz
We will have a class quiz based on our in-class work on Monday, Jan 26. Tomorrow's lesson will focus on Lewis structures and lots of practice with Bohr diagrams
Tuesday, 6 January 2015
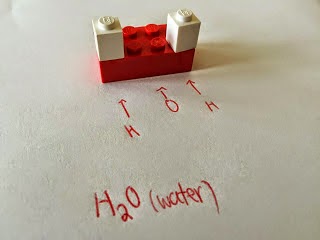
The Periodic Table: The Lego set of the Universe
The universe is made of MATTER and ENERGY.
Matter is anything with mass and volume. Everything is made of matter. All matter is made of subunits we call ATOMS, which themselves are made of subatomic particles such as:
NEUTRONS, PROTONS and ELECTRONS.
Today we sorted lego and built things and categorized what we built. This activity is meant to illustrate the big lego set of the universe, the PERIODIC TABLE is organized in these ways:
SIZE,
FAMILY
PERIOD
METALS AND NON METALS
A family - A COLUMN of elements which share similar characteristics. Each of these elements tend to react the same way. Examples of families:
Alkali Metal Family
Halogen Gas Family
Noble Gas Family
Period - A ROW of elements on the periodic table is called a period. Each of these elements have the same number of electron shells.
Metals and Nonmetals: This periodic table puts metals on one side and nonmetals on the other.
Reading: This lesson is a general intro to Chapter 4 section 4.1

Subscribe to:
Posts (Atom)