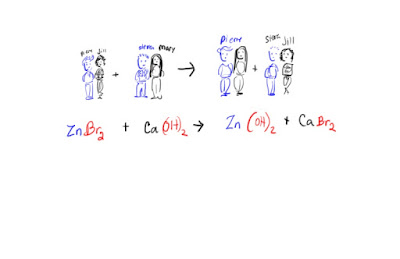Chemical
reactions may be categorized as follows
1.
Synthesis
2.
Decomposition
3.
Single replacement
4.
Double replacement
5. Combustion
6. Oxidation of metals
A Synthesis reaction:
element +
element --> compound
Decomposition reaction:
Compound --> element +
element
Or
Compound --> smaller compound + element
Combustion
Carbon based
fuel + oxygen --> carbon dioxide + water
Cell respiration looks like combustion,
BUT IT IS
NOT. YOUR CELLS ARE NOT COMBUSTING
IN THE MITOCHONDRIA
Glucose + oxygen --> carbon dioxide + water + energy for cell