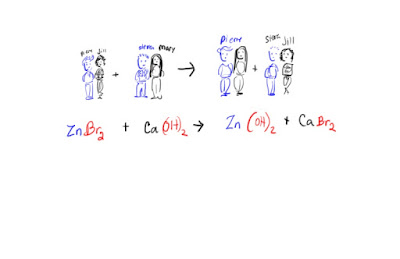I am absent today due to unexpected illness. Our Dry Ice Lab is therefore postponed to Thursday. Please open the windows and turn on the fans on low for ventilation.
Today, you will learn about the different types of reaction and review these types with the teacher.
This is a study guide and notes for different types of reactions You will work on this today.
Here are some practice problems to work on today. If you did not get the sheets in class you can do those problems to practice. This material and the study guide questions will be on this friday's quiz.
TEAMS ASSIGNMENT:
For 10 marks, make a lego model of each type of reaction. Label your lego reactions and photograph it with your maple leaf. If you are working from home, use any material you have on hand to create the models.
TEACHING NOTES:
First lets review the main types of reactions:
Chemical reactions may be categorized as follows :
Metal + oxygen --> metal oxide
ACID BASE REACTIONS ARE ALL NEUTRALIZATION REACTIONS