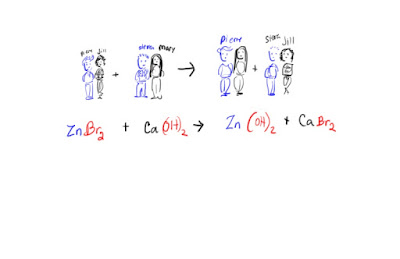The answer key to the summary notes is here
More Radiation Summary Notes ,which we will go over later, can be downloaded here
Monday, 11 December 2017
Thursday, 7 December 2017
Radioactivity
We learned about the devastating effects of the atom bomb and the theory behind the Manhattan Project. Download the presentation here. I gave you a worksheet on radiation. You can check your answers here.
Wednesday, 22 November 2017
acids bases and salts
ACIDS BASES
AND SALTS
ACID + BASE --> SALT + HOH
H-anion + cation-OH --> cation-anion + HOH
Neutralization
Acids release
H+ ions in
solution with water
Bases release OH- ions
in solution with water
The word “acidity”
refers to the concentration of
Hydrogen ions
H+ in solution. If there are more H+
Ions, then
the solution is more acidic!
The word “basic”
refers to the concentration of
Hydroxide ions
or OH- in solution. If there are
more
OH- ions,
the solution is more basic!
Acids
absorb hydroxides to neutralize a base
Bases
absorb hydrogens to neutralize an acid.
pH SCALE
A pH scale
is a way of measuring the
Concentration
of hydrogen ions in solution.
The scale
goes from 1 to 14
1 2
3 4 5
6 7 8 9
10 11 12 13
14
very neutral very
acidic basic
Acids have a pH of less than 7
Bases have a pH
of more than 7
pH 7 is H+ = OH-
every stage on the pH scale is a 10fold increase
so pH 7 has 102 more Hydrogens than pH 9.
Pure water is pH
7
Milk is pH 6
Lemons are pH2
or 3
Bleach is pH 12
Baking soda is pH
9
pH indicators
Some things change colour in the presence
Of an acid or a base
For example
Cabbage juice will turn pink for acid, blue for base
Litmus paper will turn pink for acid, blue for base
Phenolphthalein will turn pink for base and clear for
Acid
Bromothymol blue will turn blue for base and
yellow for acid
Monday, 20 November 2017
Red Wine or White Wine
We did a demo called" red wine or white wine" and did a lab where we looked at three unknown liquids.
Write a lab report due next period. It must include the following headings:
Hypothesis
Purpose
Procedure
Results
My hypothesis was proven right or wrong because....
Conclusion
Write a lab report due next period. It must include the following headings:
Hypothesis
Purpose
Procedure
Results
My hypothesis was proven right or wrong because....
Conclusion
EVALUATION IS OUT OF 25 IN TOTAL, NO MORE THAN 3 PEOPLE WRITING A COMMON REPORT
5
presentation is out of 5. for 5/5 it is typed with illustrations or photos and emailed to msngscience@gmail.com. for 4/5 it is pretty good but there are a few spelling mistakes. for 0-3 out of 5 it is in pencil or messy
10
Clear writing is out of 10. for 9/10 or 10/10, it is written with no English mistakes and it clearly communicates each part of the lab report. 7-8/10 it is very well written for 5-6/10 some parts are not so clear and the reader must guess what you are trying to say. 0-4/10, it is minimally communicating what happened
10
Paragraphs are insightful and your observations are well done. this is out of 10.
9-10/10, your observations and conclusions are well written and logical. It is brilliant
7-8/10 your observations and conclusions are very well done
5-6 your conclusions don't really logically follow from your results
0-4 your conclusions are not relating to your results at all
ACKNOWLEDGEMENTS
write all names and who contributed what including the % of their contribution. so two people who contributed equally would be50%
EMAIL IT to msngscience@gmail.com
5
presentation is out of 5. for 5/5 it is typed with illustrations or photos and emailed to msngscience@gmail.com. for 4/5 it is pretty good but there are a few spelling mistakes. for 0-3 out of 5 it is in pencil or messy
10
Clear writing is out of 10. for 9/10 or 10/10, it is written with no English mistakes and it clearly communicates each part of the lab report. 7-8/10 it is very well written for 5-6/10 some parts are not so clear and the reader must guess what you are trying to say. 0-4/10, it is minimally communicating what happened
10
Paragraphs are insightful and your observations are well done. this is out of 10.
9-10/10, your observations and conclusions are well written and logical. It is brilliant
7-8/10 your observations and conclusions are very well done
5-6 your conclusions don't really logically follow from your results
0-4 your conclusions are not relating to your results at all
ACKNOWLEDGEMENTS
write all names and who contributed what including the % of their contribution. so two people who contributed equally would be50%
EMAIL IT to msngscience@gmail.com
Naming acids
Today we reviewed naming acids and I asked you to write 5 "...ide" acids and 5 "...ite " acids and also 5 "...ate" acids. also you did an exercise in naming acids last block
Tuesday, 7 November 2017
Monday, 6 November 2017
Drawing Lewis Structures of Ionic Compounds
Today, we practiced drawing lewis structures of ionic compound Try these problems and see the answer key
Next day we will try drawing lewis structure of covalent compound. Here are the practice problems for those
Next day we will try drawing lewis structure of covalent compound. Here are the practice problems for those
Monday, 16 October 2017
practice practice practice
Recall that worksheet Chemistry practice sheet and the Chemistry practice sheet key. This was given for homework and you will be tested on this soon.
if you are done that, then there is another worksheet on types of reactions:
worksheet. Here is the answer key for types of reactions. Please write out these answers in your notebook and show me when done for work period marks. There is a typo on this one:
Section 2 number 3 has a typo on answer key. The co-efficients should be...4, 5, 4,6 and not what they have on the key
Number 3 section 3 is 1,2,2
if you are done that, then there is another worksheet on types of reactions:
worksheet. Here is the answer key for types of reactions. Please write out these answers in your notebook and show me when done for work period marks. There is a typo on this one:
Section 2 number 3 has a typo on answer key. The co-efficients should be...4, 5, 4,6 and not what they have on the key
Number 3 section 3 is 1,2,2
Thursday, 12 October 2017
Types of Chemical Reactions
Chemical
reactions may be categorized as follows
1.
Synthesis
2.
Decomposition
3.
Single replacement
4.
Double replacement
5. Combustion
6. Oxidation of metals
A Synthesis reaction:
element +
element --> compound
Decomposition reaction:
Compound --> element +
element
Or
Compound --> smaller compound + element
Combustion
Carbon based
fuel + oxygen --> carbon dioxide + water
Cell respiration looks like combustion,
BUT IT IS
NOT. YOUR CELLS ARE NOT COMBUSTING
IN THE MITOCHONDRIA
Glucose + oxygen --> carbon dioxide + water + energy for cell
balancing lego equations, practice sheets and types of reactions
We practiced balancing equations using lego. make sure you finish this and hand it in to msngscience@gmail.com
IF you are finished, practice your chemistry:
Recall that worksheet Chemistry practice sheet and the Chemistry practice sheet key. This was given for homework and you will be tested on this soon.
if you are done that, then there is another worksheet on types of reactions:
worksheet. Here is the answer key for types of reactions
IF you are finished, practice your chemistry:
Recall that worksheet Chemistry practice sheet and the Chemistry practice sheet key. This was given for homework and you will be tested on this soon.
if you are done that, then there is another worksheet on types of reactions:
worksheet. Here is the answer key for types of reactions
Tuesday, 10 October 2017
Calcium and Water lab write up
We did notes on the Law of Conservation of Mass and we did a lab. This is the criteria for the lab write up
Your lab must include the following
1. First and Last names of all investigators
2. Purpose of lab: write about how this lab illustrates the law of conservation of mass. Explain how the lab relates to the law
3. Materials: write a complete list of materials that we used
4. Procedure: write a step by step procedure (including illustrations if it makes it clearer) that is so well written that another person could read it and replicate your actions exactly
5. What is the question we are trying to answer? Write the two chemistry equations which were our possible outcomes of this reaction? Oxygen gas or Hydrogen gas?
6. Results: Describe in detail what happened after you did the procedure. Include any photos you took. How did you know you made the gas, which gas? And was it Calcium hydroxide that was made? How do you know?
7. Write your conclusion in paragraph form.
EVALUATION IS OUT OF 25 IN TOTAL
5
presentation is out of 5. for 5/5 it is typed with illustrations or photos and emailed to msngscience@gmail.com. for 4/5 it is pretty good but there are a few spelling mistakes. for 0-3 out of 5 it is in pencil or messy
10
Clear writing is out of 10. for 9/10 or 10/10, it is written with no English mistakes and it clearly communicates each part of the lab report. 7-8/10 it is very well written for 5-6/10 some parts are not so clear and the reader must guess what you are trying to say. 0-4/10, it is minimally communicating what happened
10
Paragraphs are insightful and your observations are well done. this is out of 10.
9-10/10, your observations and conclusions are well written and logical. It is brilliant
7-8/10 your observations and conclusions are very well done
5-6 your conclusions don't really logically follow from your results
0-4 your conclusions are not relating to your results at all
ACKNOWLEDGEMENTS
write all names and who contributed what including the % of their contribution. so two people who contributed equally would be50%
Your lab must include the following
1. First and Last names of all investigators
2. Purpose of lab: write about how this lab illustrates the law of conservation of mass. Explain how the lab relates to the law
3. Materials: write a complete list of materials that we used
4. Procedure: write a step by step procedure (including illustrations if it makes it clearer) that is so well written that another person could read it and replicate your actions exactly
5. What is the question we are trying to answer? Write the two chemistry equations which were our possible outcomes of this reaction? Oxygen gas or Hydrogen gas?
6. Results: Describe in detail what happened after you did the procedure. Include any photos you took. How did you know you made the gas, which gas? And was it Calcium hydroxide that was made? How do you know?
7. Write your conclusion in paragraph form.
EVALUATION IS OUT OF 25 IN TOTAL
5
presentation is out of 5. for 5/5 it is typed with illustrations or photos and emailed to msngscience@gmail.com. for 4/5 it is pretty good but there are a few spelling mistakes. for 0-3 out of 5 it is in pencil or messy
10
Clear writing is out of 10. for 9/10 or 10/10, it is written with no English mistakes and it clearly communicates each part of the lab report. 7-8/10 it is very well written for 5-6/10 some parts are not so clear and the reader must guess what you are trying to say. 0-4/10, it is minimally communicating what happened
10
Paragraphs are insightful and your observations are well done. this is out of 10.
9-10/10, your observations and conclusions are well written and logical. It is brilliant
7-8/10 your observations and conclusions are very well done
5-6 your conclusions don't really logically follow from your results
0-4 your conclusions are not relating to your results at all
ACKNOWLEDGEMENTS
write all names and who contributed what including the % of their contribution. so two people who contributed equally would be50%
Tuesday, 3 October 2017
Chemistry Practice for Chem Charate!!
A really useful Chemistry practice sheet Here is the Chemistry practice sheet key. Note there is another quiz next period
Wednesday, 27 September 2017
We went over compounds lately monatomic atoms and covalent compounds.
Now we are doing a unit on safety. Please do the reading, the homework and MAKE A POSTER SHOWING ALL THE RULES. SUMMARIZE ALL THE RULES IN 4 OR 5 STATEMENTS
Now we are doing a unit on safety. Please do the reading, the homework and MAKE A POSTER SHOWING ALL THE RULES. SUMMARIZE ALL THE RULES IN 4 OR 5 STATEMENTS
1.
WHMIS - WORKPLACE HAZARDOUS
MATERIALS INFORMATION
SYSTEM
THESE symbols are on the MSDS sheet for
all chemicals Materials safety Data Sheet.
Follow teacher instructions
WHMIS - WORKPLACE HAZARDOUS
MATERIALS INFORMATION
SYSTEM
THESE symbols are on the MSDS sheet for
all chemicals Materials safety Data Sheet.
Follow teacher instructions
a. If you wish to change instructions, check with teacher
b. If you break anything or get hurt, report it REPORT ACCIDENTS
2. No horseplay, fooling around
3. Keep yourself safe
a. Hair tied back, no loose clothing, shoes are closed
b. If chemicals get on skin, use water
c. Use eyewash if anything gets in eyes
d. Wear goggles if needed
e. If you wear contacts, inform the teacher
4. Keep your area safe
a. your work area is neat and organized
b. you have your lab instructions
c. aware of fire safety and chemical safety
5. Keep the classroom safe
a. Carry materials in a safe way
b. Aware of fire safety procedure
c. Don’t remove any experiments from the classroom
d. Don’t do experiments without first checking with teacher.
6. Hot and cold and chemical safety
a. never assume the temperature of something. Take precautions
b. hot beaker and cold beaker look identical
c. always point a test tube away from everyone
d. when smelling a chemical, waft.
You will create safety rules by reading and summarizing the safety procedures in your text. Then you will take the Finn Laboratory Saftey Test
The rules can be under the following categories:
HIGHEST PRIORITIES:
THESE RULES CAN BE SUMMED UP LIKE THIS
Talk to the instructor
KEEP ORGANIZED AND SAFE
Keep the classroom safe
Keeping your work area safe
Keep safe with your colleagues
Keep yourself safe
KNOW THE SYMBOLS
Know the correct symbols, WHIMIS symbols
COMMUNICATE AND REACT TO AN EMERGENCY
Emergency procedures for fire, toxins, spills, and evacuation
SAFETY ASSIGNMENT: I divided you into groups and talked about WHMIS. Make a poster of the 20 safety rules which I handed out in class.
Your group must draw a comic showing all the safety rules on one side of the page. put numbers to show where the rule is being applied.
RUBRIK: out of 10
YOUR POSTER IS COMPLETE SHOWING ALL 20 RULES 5 marks
it is missing a few of the rules 2-4 marks
the poster is neat and organized and easy to read 3 marks
it could be better organized and not so cluttered 1-2 marks
the poster is remarkably artistic and creative and is different in some way 2 marks
the poster is very well done and artistic 1.5 marks
the poster is artistic 1 mark
You will create safety rules by reading and summarizing the safety procedures in your text. Then you will take the Finn Laboratory Saftey Test
The rules can be under the following categories:
HIGHEST PRIORITIES:
THESE RULES CAN BE SUMMED UP LIKE THIS
Talk to the instructor
KEEP ORGANIZED AND SAFE
Keep the classroom safe
Keeping your work area safe
Keep safe with your colleagues
Keep yourself safe
KNOW THE SYMBOLS
Know the correct symbols, WHIMIS symbols
COMMUNICATE AND REACT TO AN EMERGENCY
Emergency procedures for fire, toxins, spills, and evacuation
SAFETY ASSIGNMENT: I divided you into groups and talked about WHMIS. Make a poster of the 20 safety rules which I handed out in class.
Your group must draw a comic showing all the safety rules on one side of the page. put numbers to show where the rule is being applied.
RUBRIK: out of 10
YOUR POSTER IS COMPLETE SHOWING ALL 20 RULES 5 marks
it is missing a few of the rules 2-4 marks
the poster is neat and organized and easy to read 3 marks
it could be better organized and not so cluttered 1-2 marks
the poster is remarkably artistic and creative and is different in some way 2 marks
the poster is very well done and artistic 1.5 marks
the poster is artistic 1 mark
Subscribe to:
Comments (Atom)