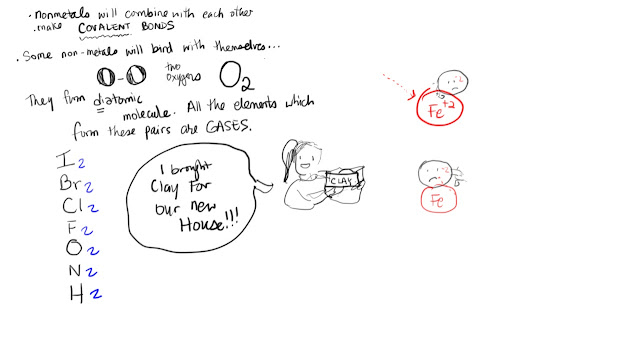
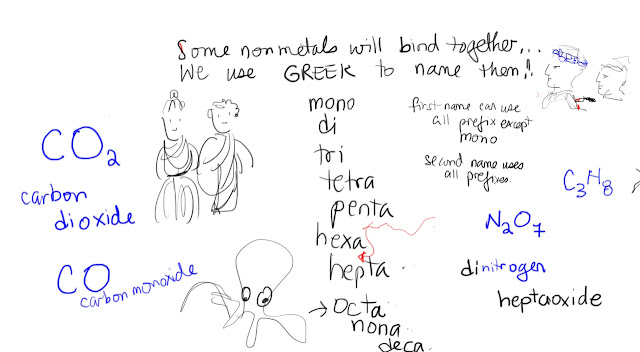
Now we are doing a unit on safety. Please do the reading, the homework and MAKE A POSTER SHOWING ALL THE RULES. SUMMARIZE ALL THE RULES IN 4 OR 5 STATEMENTS
1.
WHMIS - WORKPLACE HAZARDOUS
MATERIALS INFORMATION
SYSTEM
THESE symbols are on the MSDS sheet for
all chemicals Materials safety Data Sheet.
Follow teacher instructions
WHMIS - WORKPLACE HAZARDOUS
MATERIALS INFORMATION
SYSTEM
THESE symbols are on the MSDS sheet for
all chemicals Materials safety Data Sheet.
Follow teacher instructions
a. If you wish to change instructions, check with teacher
b. If you break anything or get hurt, report it REPORT ACCIDENTS
2. No horseplay, fooling around
3. Keep yourself safe
a. Hair tied back, no loose clothing, shoes are closed
b. If chemicals get on skin, use water
c. Use eyewash if anything gets in eyes
d. Wear goggles if needed
e. If you wear contacts, inform the teacher
4. Keep your area safe
a. your work area is neat and organized
b. you have your lab instructions
c. aware of fire safety and chemical safety
5. Keep the classroom safe
a. Carry materials in a safe way
b. Aware of fire safety procedure
c. Don’t remove any experiments from the classroom
d. Don’t do experiments without first checking with teacher.
6. Hot and cold and chemical safety
a. never assume the temperature of something. Take precautions
b. hot beaker and cold beaker look identical
c. always point a test tube away from everyone
d. when smelling a chemical, waft.
You will create safety rules by reading and summarizing the safety procedures in your text. Then you will take the Finn Laboratory Saftey Test
The rules can be under the following categories:
HIGHEST PRIORITIES:
THESE RULES CAN BE SUMMED UP LIKE THIS
Talk to the instructor
KEEP ORGANIZED AND SAFE
Keep the classroom safe
Keeping your work area safe
Keep safe with your colleagues
Keep yourself safe
KNOW THE SYMBOLS
Know the correct symbols, WHIMIS symbols
COMMUNICATE AND REACT TO AN EMERGENCY
Emergency procedures for fire, toxins, spills, and evacuation
SAFETY ASSIGNMENT: I divided you into groups and talked about WHMIS. Make a poster of the 20 safety rules which I handed out in class.
Your group must draw a comic showing all the safety rules on one side of the page. put numbers to show where the rule is being applied.
RUBRIK: out of 10
YOUR POSTER IS COMPLETE SHOWING ALL 20 RULES 5 marks
it is missing a few of the rules 2-4 marks
the poster is neat and organized and easy to read 3 marks
it could be better organized and not so cluttered 1-2 marks
the poster is remarkably artistic and creative and is different in some way 2 marks
the poster is very well done and artistic 1.5 marks
the poster is artistic 1 mark
You will create safety rules by reading and summarizing the safety procedures in your text. Then you will take the Finn Laboratory Saftey Test
The rules can be under the following categories:
HIGHEST PRIORITIES:
THESE RULES CAN BE SUMMED UP LIKE THIS
Talk to the instructor
KEEP ORGANIZED AND SAFE
Keep the classroom safe
Keeping your work area safe
Keep safe with your colleagues
Keep yourself safe
KNOW THE SYMBOLS
Know the correct symbols, WHIMIS symbols
COMMUNICATE AND REACT TO AN EMERGENCY
Emergency procedures for fire, toxins, spills, and evacuation
SAFETY ASSIGNMENT: I divided you into groups and talked about WHMIS. Make a poster of the 20 safety rules which I handed out in class.
Your group must draw a comic showing all the safety rules on one side of the page. put numbers to show where the rule is being applied.
RUBRIK: out of 10
YOUR POSTER IS COMPLETE SHOWING ALL 20 RULES 5 marks
it is missing a few of the rules 2-4 marks
the poster is neat and organized and easy to read 3 marks
it could be better organized and not so cluttered 1-2 marks
the poster is remarkably artistic and creative and is different in some way 2 marks
the poster is very well done and artistic 1.5 marks
the poster is artistic 1 mark