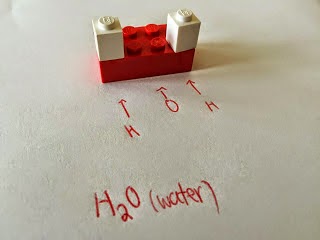
The universe is made of MATTER and ENERGY.
Matter is anything with mass and volume. Everything is made of matter. All matter is made of subunits we call
ATOMS, which themselves are made of subatomic particles such as:
NEUTRONS, PROTONS and ELECTRONS.
Today we sorted lego and built things and categorized what we built. This activity is meant to illustrate the big lego set of the universe, the
PERIODIC TABLE is organized in these ways:
SIZE,
FAMILY
PERIOD
METALS AND NON METALS
A family - A COLUMN of elements which share similar characteristics. Each of these elements tend to react the same way. Examples of families:
Alkali Metal Family
Halogen Gas Family
Noble Gas Family
Period - A ROW of elements on the periodic table is called a period. Each of these elements have the same number of electron shells.
Metals and Nonmetals: This periodic table puts
metals on one side and nonmetals on the other.
Reading: This lesson is a general intro to Chapter 4 section 4.1